The FDA recently cleared this device to treat depression (and it’s not a talk therapy)
People in treatment for major depression now have another recovery option to try.
Rejoyn, the first drug cleared by the Food and Drug Administration to help treat depression, was officially announced to the public on Tuesday.
Rejoyn is not a form of digital speech therapy or an extensive library of mental exercises, it is a form of therapy that helps people change negative thoughts and beliefs.
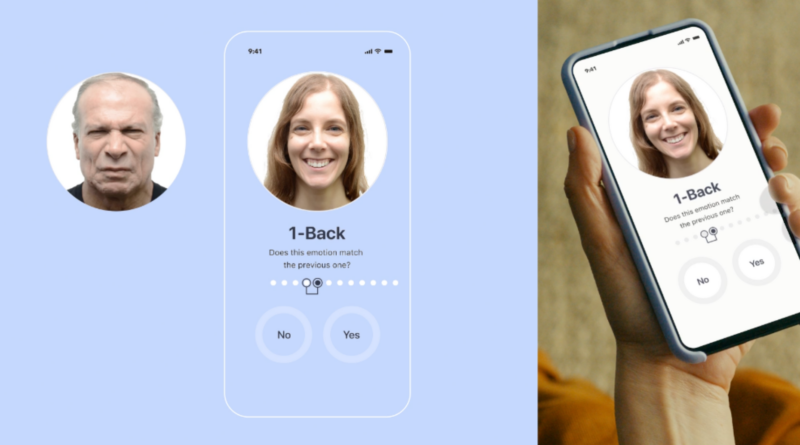
Instead, the device’s parent company, Otsuka Precision Health, likens Rejoyn to physical therapy for the brain. The program offers a six-week program that challenges patients to identify and remember four specific emotions, such as happiness and disgust, they see in a series of photographs of faces, and then decide whether they agree with the emotion. face in front of them.
A mental health coach is ready to message you right now
Known as the emotional facial memory task (EMFT), this activity is thought to help patients strengthen neural connections between the brain’s prefrontal cortex, which controls executive function, and the amygdala, which dealing with emotions.
As users improve their face matching skills, they move on to more difficult face matching levels.
Rejoyn appears to work by strengthening nerve connections in the brain.
Credit: Otsuka Pharmaceutical
“EMFT is designed to strengthen circuits that have been shown to be dysfunctional in stress,” Dr. John Kraus, Otsuka Pharmaceutical’s chief medical officer, told Mashable. Rejoyn also includes short courses in cognitive behavioral therapy.
Although Rejoyn is now available on the App Store and Google Play, only patients with major depression who are currently taking antidepressants are eligible for a prescription for the app. Patients must also be at least 22 years old.
Mashable Top Stories
Rejoyn is considered low risk by the FDA. Kraus said the company saw no adverse events during its clinical trial, but as with any treatment for depression, patients should contact their healthcare provider if their symptoms worsen. not improving, or have suicidal thoughts or behavior.
Rejoyn was tested against another memory task involving shapes rather than faces. Those who resembled faces saw an improvement in their depression symptoms compared to those who worked with shapes. However, Rejoyn has not been tested against proven over-the-counter treatments for depression, such as cognitive behavioral therapy, which is often free.
Initially, Rejoyn will cost $50 for individual paying patients. For insurers, the price is $200. Otsuka Precision Health expects insurance carriers to eventually add coverage for the device. Patients can obtain a prescription from their health care provider.
They can also request a reality check through a group of telehealth providers Wheel Health, Inc., through the Rejoyn website. The consultation, which costs $29, will assess whether Rejoyn is right for the patient. When they get a prescription, they get a unique code to access the app’s treatment program.
When the six-week treatment period ends, patients can receive cognitive behavioral therapy sessions for another four weeks. After that, they can no longer use the app. Kraus said patients can get a new prescription in the future, if recommended by their health care provider.
Although Rejoyn does not emphasize success, it requires consistent and consistent care. Each similar task takes 20 to 30 minutes. Users can stop their progress if they need to leave for a while, but the program will start after 15 minutes.
Because of the cost and time involved, patients may want to consider whether the device is the best fit for their needs, compared to other devices or treatments, said Dr. John Torous, psychiatrist and director of digital psychiatry at Beth Israel Deaconess Medical. Boston Center.
He noted that many people stop interacting with mental health tools within weeks of using them, so patients can benefit greatly from choosing a tool they believe they will stick with. from the beginning.
Torous said patients are more likely to experience progress when using the app as part of ongoing treatment with a mental health provider.
“It’s hard to help yourself, but at least get your doctor to check…[and] keeping you accountable is going to be critical to making it work,” Torous said.
If you are feeling suicidal or have a mental health problem, please talk to someone. You can reach the 988 Suicide and Crisis Lifeline at 988; Trans Lifeline at 877-565-8860; or the Trevor Project at 866-488-7386. Text “START” on the Crisis Text Line at 741-741. Contact the NAMI HelpLine at 1-800-950-NAMI, Monday through Friday from 10:00 am – 10:00 pm ET, or email [email protected]. If you don’t like the phone, consider using the 988 Suicide and Crisis Lifeline Chat on crisischat.org. Here is a list of international sources.
Heads
Tools & Software Community Benefit
#FDA #cleared #device #treat #depression #talk #therapy